Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Pu
Pu O
O powder using master sintering curve theory
powder using master sintering curve theoryNakamichi, Shinya; Sunaoshi, Takeo*; Hirooka, Shun; Vauchy, R.; Murakami, Tatsutoshi
Journal of Nuclear Materials, 595, p.155072_1 - 155072_11, 2024/07
Irisawa, Eriko; Kato, Chiaki
Journal of Nuclear Materials, 591, p.154914_1 - 154914_10, 2024/04
Times Cited Count:0The amount of corrosion of austenitic stainless-steel R-SUS304ULC was evaluated considering the changes in solution composition and boiling during actual concentration operations. Austenitic stainless-steel R-SUS304ULC is the structural material of the highly radioactive liquid waste concentrator in Japanese spent fuel reprocessing plant, which treats highly corrosive nitric acid solutions during enrichment operations. The study results show that it is necessary to focus on nitric acid concentrations, oxidizing metal ion concentrations, and decompression boiling as factors that accelerate the corrosion rate of stainless steel because of cathodic reaction activation.
Horii, Yuta; Hirooka, Shun; Uno, Hiroki*; Ogasawara, Masahiro*; Tamura, Tetsuya*; Yamada, Tadahisa*; Furusawa, Naoya*; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 588, p.154799_1 - 154799_20, 2024/01
Times Cited Count:1 Percentile:72.91(Materials Science, Multidisciplinary)The thermal conductivities of near-stoichiometric (U,Pu,Am)O doped with Nd
doped with Nd O
O /Sm
/Sm O
O , which is major fission product (FP) generated by a uranium-plutonium mixed oxides (MOX) fuel irradiation, as simulated fission products are evaluated at 1073-1673 K. The thermal conductivities are calculated from the thermal diffusivities that are measured using the laser flash method. To evaluate the thermal conductivity from a homogeneity viewpoint of Nd/Sm cations in MOX, the specimens with different homogeneity of Nd/Sm are prepared using two kinds of powder made by ball-mill and fusion methods. A homogeneous Nd/Sm distribution decreases the thermal conductivity of MOX with increasing Nd/Sm content, whereas heterogeneous Nd/Sm has no influence. The effect of Nd/Sm on the thermal conductivity is studied using the classical phonon transport model (A+BT)
, which is major fission product (FP) generated by a uranium-plutonium mixed oxides (MOX) fuel irradiation, as simulated fission products are evaluated at 1073-1673 K. The thermal conductivities are calculated from the thermal diffusivities that are measured using the laser flash method. To evaluate the thermal conductivity from a homogeneity viewpoint of Nd/Sm cations in MOX, the specimens with different homogeneity of Nd/Sm are prepared using two kinds of powder made by ball-mill and fusion methods. A homogeneous Nd/Sm distribution decreases the thermal conductivity of MOX with increasing Nd/Sm content, whereas heterogeneous Nd/Sm has no influence. The effect of Nd/Sm on the thermal conductivity is studied using the classical phonon transport model (A+BT) . The dependences of the coefficients A and B on the Nd/Sm content (C
. The dependences of the coefficients A and B on the Nd/Sm content (C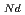 and C
and C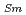 , respectively) are evaluated as: A(mK/W)=1.70
, respectively) are evaluated as: A(mK/W)=1.70  10
10 + 0.93C
+ 0.93C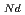 + 1.20C
+ 1.20C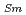 , B(m/W)=2.39
, B(m/W)=2.39  10
10 .
.
 mixed oxides
mixed oxidesVauchy, R.; Matsumoto, Taku; Hirooka, Shun; Uno, Hiroki*; Tamura, Tetsuya*; Arima, Tatsumi*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Nakamura, Hiroki; Machida, Masahiko; et al.
Journal of Nuclear Materials, 588, p.154786_1 - 154786_13, 2024/01
Times Cited Count:1 Percentile:72.91(Materials Science, Multidisciplinary)Narukawa, Takafumi; Kondo, Keietsu; Fujimura, Yuki; Kakiuchi, Kazuo; Udagawa, Yutaka; Nemoto, Yoshiyuki
Journal of Nuclear Materials, 587, p.154736_1 - 154736_8, 2023/12
Times Cited Count:1 Percentile:0.01(Materials Science, Multidisciplinary)Watanabe, So; Takahatake, Yoko; Ogi, Hiromichi*; Osugi, Takeshi; Taniguchi, Takumi; Sato, Junya; Arai, Tsuyoshi*; Kajinami, Akihiko*
Journal of Nuclear Materials, 585, p.154610_1 - 154610_6, 2023/11
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)Vauchy, R.; Hirooka, Shun; Watanabe, Masashi; Yokoyama, Keisuke; Murakami, Tatsutoshi
Journal of Nuclear Materials, 584, p.154576_1 - 154576_11, 2023/10
Times Cited Count:2 Percentile:90.12(Materials Science, Multidisciplinary)Ukai, Shigeharu; Sakamoto, Kan*; Otsuka, Satoshi; Yamashita, Shinichiro; Kimura, Akihiko*
Journal of Nuclear Materials, 583, p.154508_1 - 154508_24, 2023/09
Times Cited Count:5 Percentile:94.3(Materials Science, Multidisciplinary)Narukawa, Takafumi; Kondo, Keietsu; Fujimura, Yuki; Kakiuchi, Kazuo; Udagawa, Yutaka; Nemoto, Yoshiyuki
Journal of Nuclear Materials, 582, p.154467_1 - 154467_12, 2023/08
Times Cited Count:3 Percentile:95.99(Materials Science, Multidisciplinary)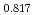 Pu
Pu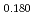 Am
Am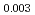 O
O uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 K
uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 KVauchy, R.; Sunaoshi, Takeo*; Hirooka, Shun; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 580, p.154416_1 - 154416_11, 2023/07
Times Cited Count:4 Percentile:98.08(Materials Science, Multidisciplinary)Kitagaki, Toru; Krasnov, V.*; Ikeda, Atsushi
Journal of Nuclear Materials, 576, p.154224_1 - 154224_14, 2023/04
Times Cited Count:1 Percentile:53.89(Materials Science, Multidisciplinary)Nemoto, Yoshiyuki; Ishijima, Yasuhiro; Kondo, Keietsu; Fujimura, Yuki; Kaji, Yoshiyuki
Journal of Nuclear Materials, 575, p.154209_1 - 154209_19, 2023/03
Times Cited Count:1 Percentile:31.61(Materials Science, Multidisciplinary)Previous studies had shown that in certain conditions, the rate of oxidation of zirconium (Zr) based alloy fuel cladding is higher in air-steam mixtures than in dry air. In severe accidents in the spent fuel pool and in other air ingress accidents in nuclear power plants, the cladding is likely to be oxidized in an air-steam mixture, which makes it crucial to have an in-depth understanding of the nature of oxidation and its kinetics in that environment. Oxidation tests were conducted at 800 C on Zircaloy-4 specimens in a mix of (air+steam) with various component ratios. Oxidation kinetics, details of the oxide layer, and hydrogen pick-up in the specimen were studied to investigate the mechanism of oxidation in each of these sets of conditions. Zirconium nitride precipitation in the oxide layer during the initial stages of the pre-breakaway oxidation stage and the widespread porous oxide growth on the cladding surface in the latter post-BA oxidation stage are related to the oxidation mechanism in the air-steam mixture. The differences in the mechanism of oxidation of the cladding in dry air and air-steam mixtures are discussed based on the experimental results.
C on Zircaloy-4 specimens in a mix of (air+steam) with various component ratios. Oxidation kinetics, details of the oxide layer, and hydrogen pick-up in the specimen were studied to investigate the mechanism of oxidation in each of these sets of conditions. Zirconium nitride precipitation in the oxide layer during the initial stages of the pre-breakaway oxidation stage and the widespread porous oxide growth on the cladding surface in the latter post-BA oxidation stage are related to the oxidation mechanism in the air-steam mixture. The differences in the mechanism of oxidation of the cladding in dry air and air-steam mixtures are discussed based on the experimental results.
 C
CMiyazawa, Takeshi; Kikuchi, Yuta*; Ando, Masami*; Yu, J.-H.*; Yabuuchi, Kiyohiro*; Nozawa, Takashi*; Tanigawa, Hiroyasu*; Nogami, Shuhei*; Hasegawa, Akira*
Journal of Nuclear Materials, 575, p.154239_1 - 154239_11, 2023/03
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)Yabuuchi, Kiyohiro*; Suzudo, Tomoaki
Journal of Nuclear Materials, 574, p.154161_1 - 154161_6, 2023/02
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)In nuclear materials, irradiation defects cause degradation of mechanical properties. In these materials, the relationship between dislocations and voids is particularly important for mechanical strength. Although only spherical voids have been studied in the past, this study focuses on faceted voids, which are observed simultaneously with spherical voids. In the current study, molecular dynamics was used to analyze the effect of faceted voids in the irradiation hardening of pure iron. Specifically, we clarified the difference in obstacle strength and interaction processes between spherical voids and faceted voids, and that even faceted voids show differences in interaction depending on their crystallographic arrangement with dislocations.
Kakiuchi, Kazuo; Amaya, Masaki; Udagawa, Yutaka
Journal of Nuclear Materials, 573, p.154110_1 - 154110_7, 2023/01
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)Oka, Hiroshi*; Tanno, Takashi; Yano, Yasuhide; Otsuka, Satoshi; Kaito, Takeji; Hashimoto, Naoyuki*
Journal of Nuclear Materials, 572, p.154032_1 - 154032_8, 2022/12
Times Cited Count:3 Percentile:68.71(Materials Science, Multidisciplinary)9Cr oxide dispersion strengthened steels with slightly different nitrogen concentrations (0.0034 - 0.029 wt%) were prepared and their creep property at 973 K was investigated with microstructural characterization before and after the creep test. The creep strength decreased significantly as the nitrogen concentration increased. Microstructural observation revealed that, in the higher nitrogen concentration specimen, coarse Y-rich inclusions were found along the boundary between transformed ferrite region and residual ferrite region. The solubility difference of nitrogen in  and
and  phase would induce the localized increment of nitrogen concentration in the boundary region during the austenitizing process, resulting in the thermodynamic destabilization and subsequent coarsening of the dispersed oxide particles. The rows of creep voids were found near the rupture part of the crept specimen, suggesting that the coarse inclusions were the starting point of creep void formation and the subsequent premature fracture.
phase would induce the localized increment of nitrogen concentration in the boundary region during the austenitizing process, resulting in the thermodynamic destabilization and subsequent coarsening of the dispersed oxide particles. The rows of creep voids were found near the rupture part of the crept specimen, suggesting that the coarse inclusions were the starting point of creep void formation and the subsequent premature fracture.
 Pu
Pu )
) Gd
Gd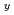 O
O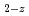 derived from molecular dynamics
derived from molecular dynamicsGalvin, C. O. T.*; Machida, Masahiko; Nakamura, Hiroki; Andersson, D. A.*; Cooper, M. W. D.*
Journal of Nuclear Materials, 572, p.154028_1 - 154028_8, 2022/12
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)UO is the primary conventional fuel used in most nuclear reactors with Gd
is the primary conventional fuel used in most nuclear reactors with Gd O
O commonly added as a burnable absorber to produce a more level power distribution in the reactor core at the beginning of operation. It can also be mixed with other actinide oxides to produce mixed oxide (MOx) fuel. In this study, molecular dynamics simulations were used to predict the specific heat capacity of Gd-doped PuO
commonly added as a burnable absorber to produce a more level power distribution in the reactor core at the beginning of operation. It can also be mixed with other actinide oxides to produce mixed oxide (MOx) fuel. In this study, molecular dynamics simulations were used to predict the specific heat capacity of Gd-doped PuO , UO
, UO and (U,Pu)O
and (U,Pu)O MOx accommodating Gd
MOx accommodating Gd substituted at cation sites via two charge compensation mechanisms - oxygen vacancy formation and the oxidation of U
substituted at cation sites via two charge compensation mechanisms - oxygen vacancy formation and the oxidation of U to U
to U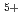 . The specific heat capacity values for PuO
. The specific heat capacity values for PuO and UO
and UO are in good agreement with other studies showing a distinct peak at high temperatures - above 1800 K. As Gd
are in good agreement with other studies showing a distinct peak at high temperatures - above 1800 K. As Gd is added, the peak height reduces for each composition considered. An analytical fit was applied to the data where Gd
is added, the peak height reduces for each composition considered. An analytical fit was applied to the data where Gd was fully charge compensated by either oxygen vacancies or U
was fully charge compensated by either oxygen vacancies or U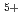 . The expression was then validated by predicting the specific heat capacity for three compositions of (U
. The expression was then validated by predicting the specific heat capacity for three compositions of (U Pu
Pu )
) Gd
Gd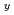 O
O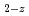 containing both oxygen vacancies and U
containing both oxygen vacancies and U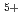 , and compared to molecular dynamics data.
, and compared to molecular dynamics data.
Okamoto, Yoshihiro; Shiwaku, Hideaki; Shimamura, Keisuke*; Kobayashi, Hidekazu; Nagai, Takayuki; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*
Journal of Nuclear Materials, 570, p.153962_1 - 153962_13, 2022/11
Simulated nuclear waste glass samples containing phosphorus, which increase the solubility of molybdenum, were prepared and analyzed using synchrotron X-ray Absorption Fine Structure (XAFS) analysis for some constituent elements and Raman spectroscopic analysis of their complex structure. Changes in local structure and chemical state due to different phosphorus additions and waste loading rates were systematically studied. Consequently, no crystalline phase due to the molybdate compound was observed even at a maximum waste content of 30 wt% (corresponding to 1.87 mol% MoO ). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO
). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO structural unit and may deprive the alkali metal coordinated to the molybdate ion.
structural unit and may deprive the alkali metal coordinated to the molybdate ion.
Ito, Daisuke*; Sato, Hirotaka*; Odaira, Naoya*; Saito, Yasushi*; Parker, J. D.*; Shinohara, Takenao; Kai, Tetsuya; Oikawa, Kenichi
Journal of Nuclear Materials, 569, p.153921_1 - 153921_6, 2022/10
Times Cited Count:1 Percentile:31.61(Materials Science, Multidisciplinary) and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:3 Percentile:68.71(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U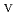
 Fe
Fe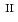
 U
U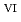 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.